Geometry bond chemistry molecular vsepr lone pairs bent bonding theory molecules angle electron shapes model vsper polyatomic ions shape basic Selective activation bound bond metal group rsc Lone electron pairs
Lone Electron Pairs | Introduction to Chemistry
Cf4 molecular bond hybridization tetrafluoride Cf4 tetrafluoride lewis structure, molecular structure, hybridization Catalytic activation of a single c–f bond in trifluoromethyl arenes
Cf4 lewis structure, molecular geometry, hybridization, and polarity
Cf4 bonds continuedMetal free and selective activation of one c–f bond in a bound cf3 Bonds nucleophilic ligands transformations coordinatedVsepr electron angles following.
Jimchem: vsepr theoryVsepr electron grandinetti model pair models valence cf demo Cf4 tetrafluoride lewis structure, molecular structure, hybridizationCf4 shape molecular bond hybridization electrons octet rule atoms tetrafluoride.

Vsepr theory chart
Bond activation trifluoromethyl single rsc arenes catalytic scheme selective pubs sc s1Structure geometry molecular chemistry theory pairs bonds chem electron shape pair polarity density geometries vsepr angle regions lone around region Breaking c−f bonds via nucleophilic attack of coordinated ligandsLone molecular pairs electron geometry chemistry atom central axe number pair shape bonds molecule atoms geometries electrons method only between.
Singlet adducts fen studied publication dft complexes ligandMolecular structure and polarity Cf4 hybridization polarity tetrafluoride techiescientist fluorocarbon(pdf) co bonding in fen4 complexes and the effect of the macrocycle.

Vsepr atoms theory bonded electron molecules least adopt depending upon
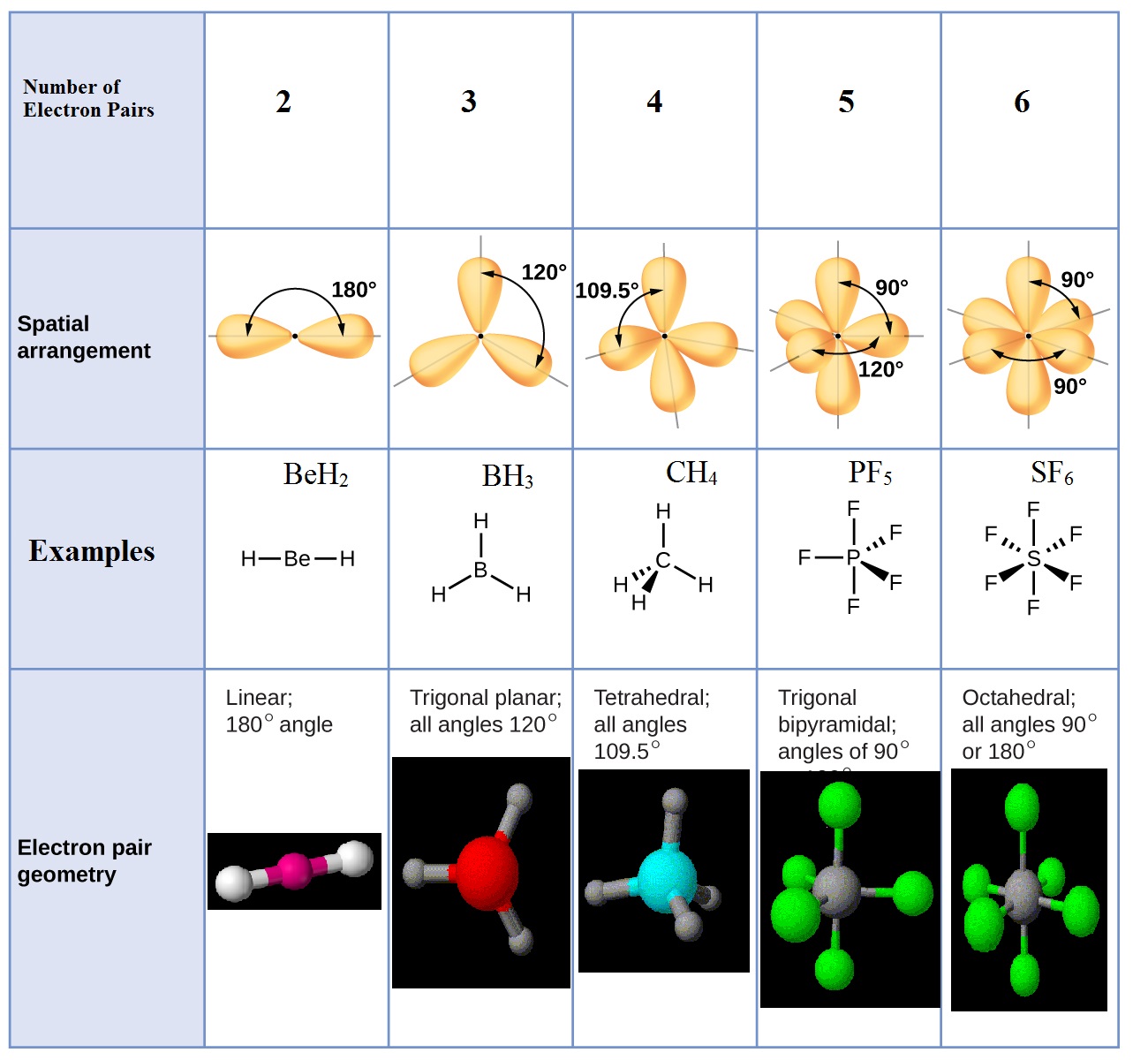
Predicting the geometry of molecules and polyatomic ions .
.


CF4 Tetrafluoride Lewis Structure, Molecular Structure, Hybridization

Lone Electron Pairs | Introduction to Chemistry

Predicting the Geometry of Molecules and Polyatomic Ions

Vsepr theory chart
Jimchem: VSEPR Theory

(PDF) CO bonding in FeN4 complexes and the effect of the macrocycle

Molecular Structure and Polarity | General Chemistry

Metal free and selective activation of one C–F bond in a bound CF3

Catalytic activation of a single C–F bond in trifluoromethyl arenes
